
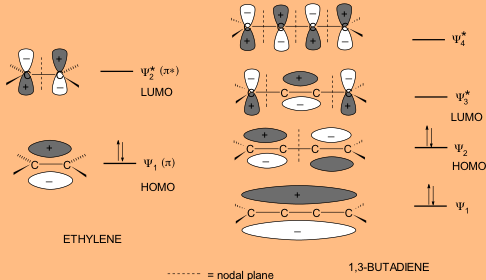

This is exemplified by formation of stable NaVO3 complex compound by interacting alkali Na atom with VO3 superhalogen. electrochemically, or from alkali metalammonia to produce ketyl radical. The interaction of VOn superhalogen with appropriate alkali metal is stronger than traditional alkali halides. Theoretical calculations confirmed the importance of the HOMOLUMO gap for this. 1, and the same situations for the frontier molecular orbital energy (E HOMO and E LUMO) levels in Table 2, clearly indicate that more active and strong excitations could be achieved in. The electron affinity of VOn suggests that these species behave as superhalogen for n≥3, which become as large as 4.52 eV for n = 5. The HOMO and LUMO have -and -like characters, respectively, for both Py and BgP. Moreover, the relative higher distribution of the electron cloud density in the HOMO and LUMO Orbitals for the precursor than those of its target dye in Fig. It is shown that a V binds with four O atoms stably such that the maximum possible oxi dation state of V can be as high as +7. Ībstract: We perform density functional calculations on the ground state geometries of VOn (n = 1–5) complexes and analyze their stability against dissociation to O atom and O2 molecule in neutral as well as anionic forms. Neeraj Misra, Department of Physics, University of Lucknow, Lucknow-226007, India. A visual inspection of the orbitals shows that the nitrogen atom of the ammonia molecule were to approach the boron atom in boron trichloride, there would be constructive (green-red) overlap. Additionally, computations of HOMO/LUMO energy level for single molecules. Authors: Shukla, Dharmesh Vikram | Srivastava, Ambrish Kumar | Misra, Neeraj *Īffiliations: Department of Physics, University of Lucknow, Lucknow, IndiaĬorresponding author. The smaller energy difference is between the ammonia HOMO and boron trichloride LUMO. Also structures of nitrogen, ammonia and carbon dioxide molecules present in.


 0 kommentar(er)
0 kommentar(er)
